Clicking any of the pictures below will let you view the structure using a Java applet
All amino acids have a common structure - they contain a central carbon atom (the 'alpha-carbon') to which is attached an amino group (-NH2), a carboxylate group (-COOH), a hydrogen, and a sidechain (or 'R-group') which varies between the amino acids. The simplest amino acids, glycine, has a second hydrogen as its R-group and is therefore the only amino acid not to have stereo-isomers.
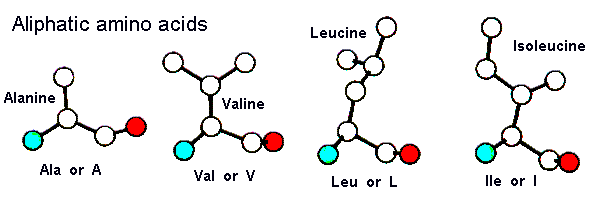
The aliphatic amino acids all contain only carbons in their sidechains. All are hydrophobic - they prefer to pack away from water and frequently form the core of proteins.
 |
 |
 |
 |
| Alanine | Valine | Leucine | Isoleucine |
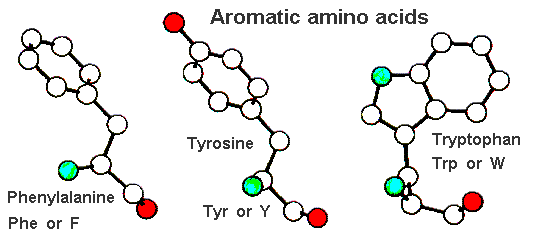
The aromatic amino acids all have aromatic rings in their sidechains. (Aromatic rings are similar to benzene and have delocalized electrons and partial double bond character.) Tyrosine and tryptophan both have some hydrophilic character (containing oxygen or nitrogen in the sidechain), while phenyl-alanine is completely non-polar. (Note, however, that the delocalized electrons of the ring can make semi-polar interactions.)
Like the aliphatic sidechains, these are largely hydrophobic and are found buried in the core of proteins. However, tyrosine often is partially exposed.
 |
 |
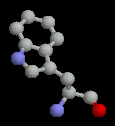 |
| Phenylalanine | Tyrosine | Tryptophan |
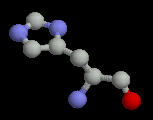
The basic amino acids all have positively charged sidechains. The pKa of histidine is around 6 and is therefore neutral at physiological pH. However, in localized environments within proteins, it may be charged and it can act as an acid-base catalyst.
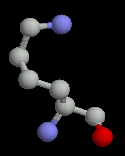
Arginine and lysine are generally found on the surface of proteins. When they are found buried, they are always making a charge-charge interaction with an acidic sidechain (aspartate or glutamate).
 |
 |
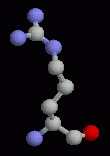 |
| Histidine | Lysine | Arginine |
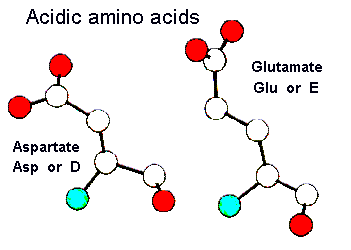
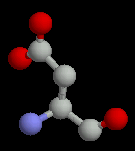
The acidic sidechains are negatively charged. Like the basic amino acids, they are generally found on the surface of proteins, but when found buried, they form charge-charge interactions with a basic amino acid.
 |
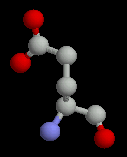 |
| Aspartate | Glutamate |
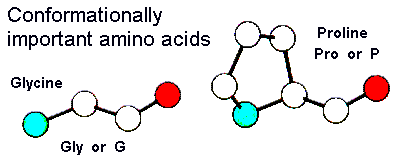
Glycine is the only amino acid not to have a sidechain, having a second hydrogen instead. It therefore does not have stereo-isomers. The lack of a sidechain allows more conformation flexibility of the backbone of the protein.
Proline is strictly an 'imino acid' rather than an amino acid. The sidechain is cyclic, binding back to the nitrogen of the backbone. This ring structure restricts the conformational flexibility of the backbone.
The peptide bonds linking amino acids have a semi-double-bond character and are therefore restricted to cis or trans conformations. In most cases peptide bonds are trans, the exception being the peptide bond preceeding a proline which is found in the cis conformation for around half of prolines.
 |
 |
| Glycine | Proline |
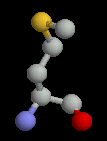
Two of the amino acids, cysteine and methionine contain sulphur. There is some debate about whether sulphur can form hydrogen bonds. It is generally accepted that it cannot, but occassionally these amino acids occur in locations where they do appear to be making a hydrogen bond. Cysteine and methionine have generally hydrophobic chacteristics.
Cysteine also has the ability to form disulphide (-S-S-) bonds. These occur when cysteine residues that are not adjacent to one another in the primary sequence come together in three dimensions.
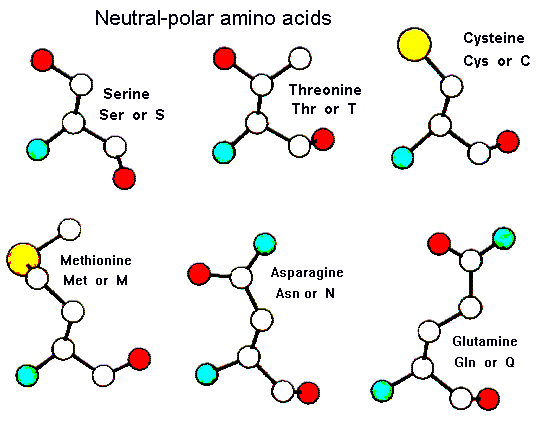
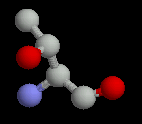
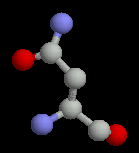
The polar amino acids all have hydroxyl (-OH) or amide (-C=ONH) groups in their sidechains. These frequently occur on the surface of proteins; when they are buried, the hydrogen bonding potential of these sidechains must be satisfied for stability.
 |
 |
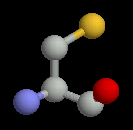 |
| Serine | Threonine | Cysteine |
 |
 |
 |
| Methionine | Asparagine | Glutamine |
Cartoon images were generated by Jon Cooper and are available at http://www.cryst.bbk.ac.uk/pps97/course/section2/SideChains/primary1.html